TEAM LEADERS : Eric Camerer and Anne Eichmann
Mail : eric.camerer@inserm.fr
CAMERER: +33 1 53 98 80 48
Localisation :
Camerer lab: 3rd floor, room 361
DOCTORAL SCHOOL : Ecole Doctorale Bio Sorbonne Paris Cité (BioSPC)

Aims
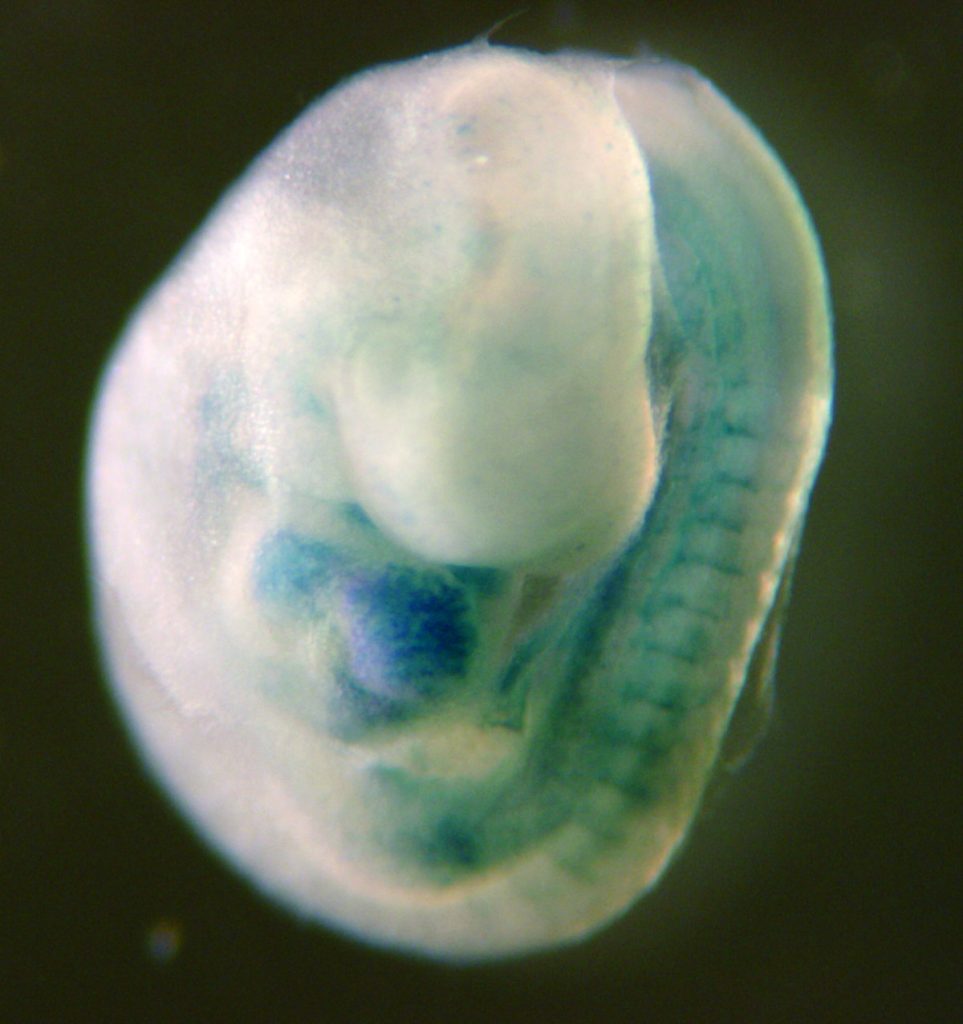
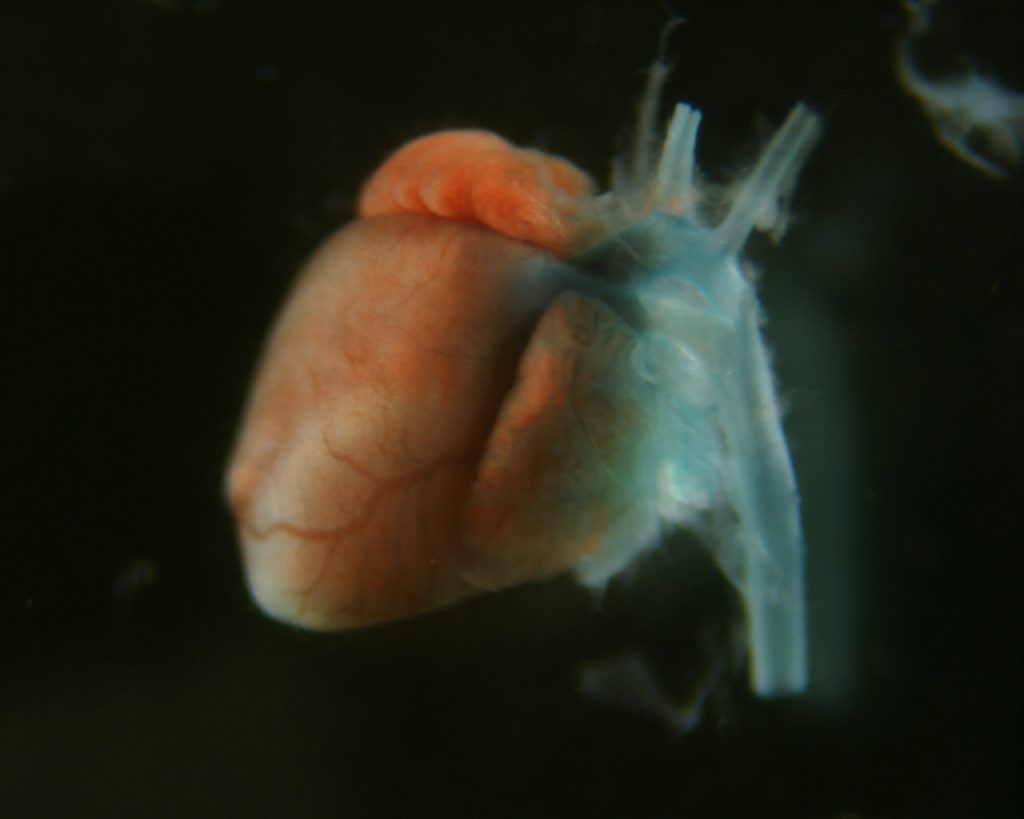
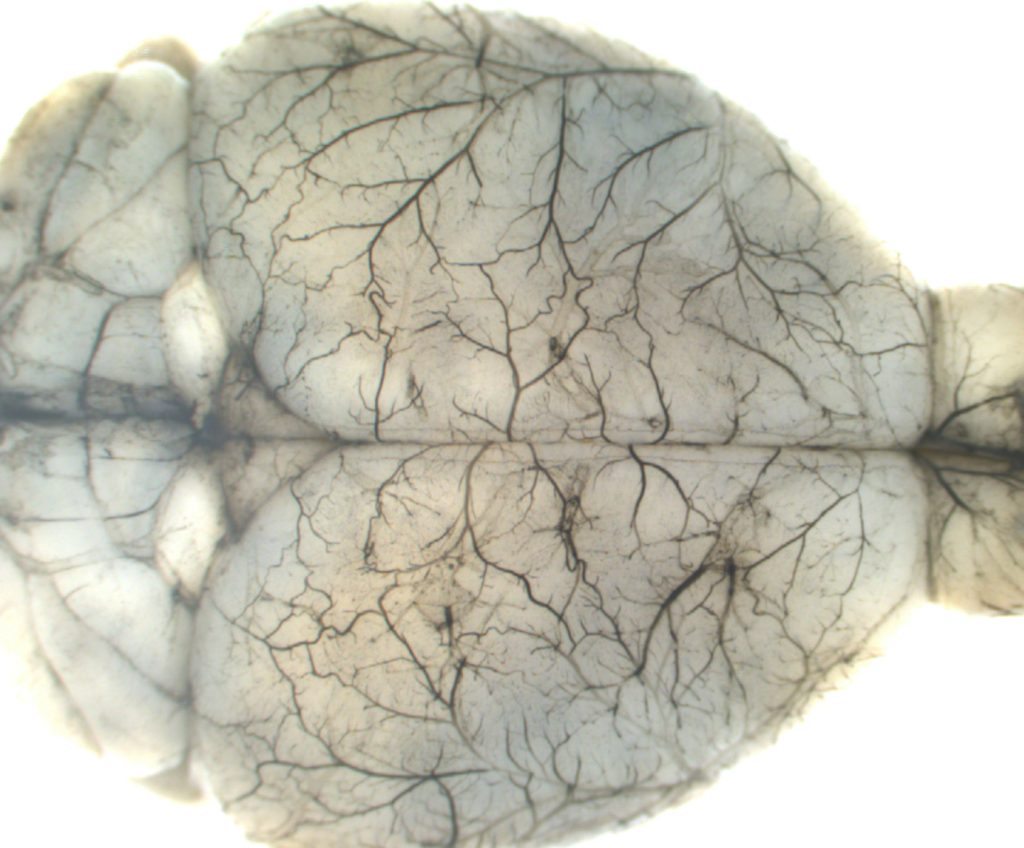
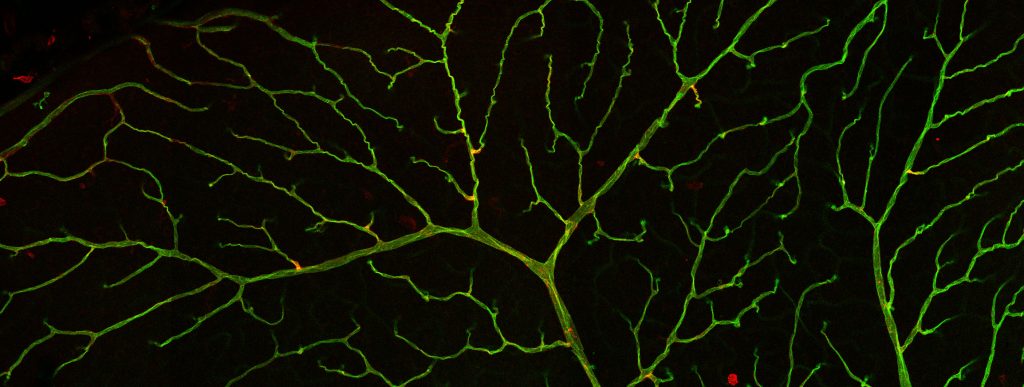
The Camerer group studies G protein-Coupled Receptor (GPCR) signaling in development and disease. Serine proteases and bioactive lipids play important roles during embryonic development that are mediated by dedicated GPCRs. Although our understanding of these GPCR families is rapidly evolving, much remains to be known about how they help coordinate vascular development and to what extent they also regulate vascular homeostasis and/or contribute to vascular disease if deregulated. Our group explores the functional domains of protease-activated receptors (PARs) and sphingosine-1-phospate receptors (S1PRs), their mechanisms of action and their interactions during development and disease. To do so, we seek to identify the sources and repertoires of their agonists to understand how and when they are mobilized, their downstream coupling to understand what messages they transmit, and the effect of gain and loss of function on cell behavior and organ function to understand their interactions and biological roles. We are particularly interested in how these receptor families work together to regulate vascular, epithelial and feto-maternal barriers.
Approximately one third of all approved drugs target GPCRs, and both PARs and S1PRs are targets for drugs in clinical use. Our research aims to discover new applications for these and other emerging drugs in the field and to inform on how therapeutic targeting of PARs and S1PRs can be refined to improve efficacy and limit side effects.