


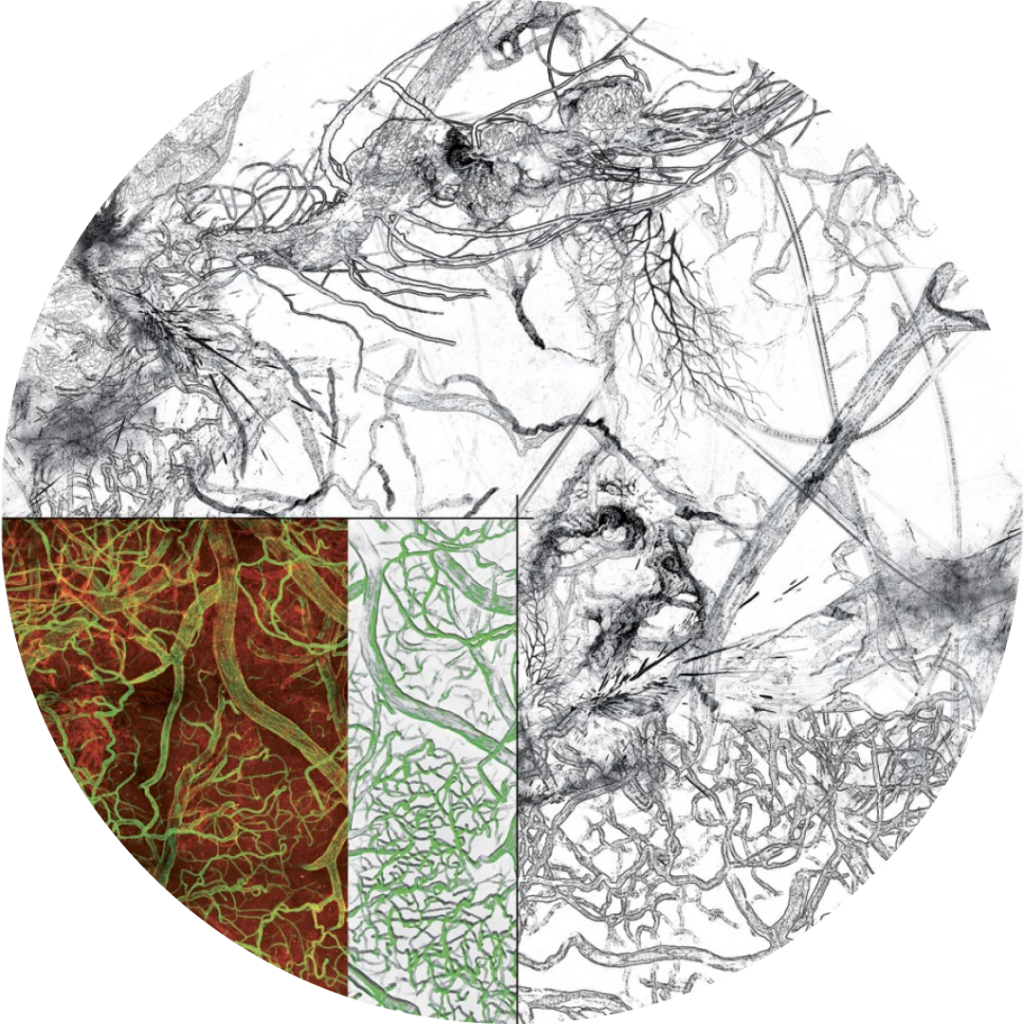

Research in our team concerns vascular system development and contribution to human pathologies. Endothelial cells (ECs) that constitute the vascular system proliferate and differentiate at the same rate as the organism grows, to ensure oxygen and nutriment supply of tissues and cells. In adults, vascular development becomes quiescent and EC proliferation occurs only in certain physiological situations (wound healing, physical exercise, pregnancy) and pathologies, including diabetes, ageing-associated macular degeneration, tissue ischemia (heart, brain, legs) and malignant tumors.
The long-term goal of the laboratory is to understand the cellular and molecular mechanisms controlling vascular patterning and contributing to vascular homeostasis in the mature organism. We aim to identify key molecular events underlying angiogenesis, lymphangiogenesis and arteriogenesis and to establish the principles governing development of the vertebrate vascular system. Vessel networks are vulnerable to diseases, and abnormal vascular development in cardiovascular disease, cancer and diabetes is the major cause of morbidity and mortality in the developed world. We expect that understanding mechanisms controlling developmental vessel patterning will lead to novel strategies to prevent these diseases.

1. Guidance of vascular patterning and vascular malformations
(Park et al. Circulation 2021; Genet G. et al. Nat. Comm. 2019; Ola R. et al. Circulation 2018; Ola R. et al. Nat. Comm 2016; Aspalter IM et al. Nat. Comm 2015; Larivée B. et al. Dev. Cell 2012; Del Toro R. et al. Blood 2010)
We have shown that in blood vessels, specialized endothelial cells called tip cells located at the extremities of growing capillary sprouts mediate guided vascular patterning. Tip cells exhibit characteristic features, including extension of filopodia that explore the tip cell environment, lack of a lumen and a slow proliferation rate. Following behind tip cells, other endothelial cells termed stalk cells form the capillary lumen and proliferate. Tip cell selection is induced by VEGF signaling through VEGFR2 and is suppressed in stalk cells by Delta- Notch and BMP9-Alk1 signaling. These discoveries have profoundly affected scientific thinking about vascular patterning and reveals novel signaling pathway that could be targeted in patients with vascular malformations such as hereditary hemorrhagic telangiectasia.
2. Guidance receptor signaling in physiological and pathological angiogenesis
(Geraldo L. et al. JCI 2021; Zhang F. et al. Nat. Comm 2016; Rama N. et al. Nat. Medicine 2015; Koch AW. et al. Dev. Cell 2011; Lu X. et al. Nature 2004)
We have shown that capillary sprouting shows morphological similarities to axon guidance. Like endothelial tip cells, axonal growth cones extend filopodia that sense and respond to extracellular guidance cues. My lab has identified several key molecules regulating capillary guidance that also function in axonal guidance, including UNC5B, a receptor for Netrin1 and Robo4 and Slit2 signaling through Robo1 and 2. Molecules regulating capillary patterning and guidance in development also operate during pathological angiogenesis and therefore represent targets for promoting or inhibiting conditions associated with aberrant vessel growth.
3. Lymphatic drainage of fluids, macromolecules and immune cells
(Jacob L. et al. J. Exp. Med 2022; Jacob et al. Nat. Comm 2019; Zhang F et al. Science 2018; Bouvrée K. et al. Circulation Research 2012; Xu Y. et al. Journal of Cell Biology 2010).
The lymphatic vasculature regulates fluid homeostasis, absorption of dietary lipids and immune system function. The absence of lymphatic vessels is lethal, and impaired lymphatic vessel development causes fluid and protein accumulation in the interstitium, resulting in lymphedema and a persistent inflammatory response. Lymphedema and chronic inflammation are aggravating factors in cardiovascular disease, and are progressive and lifelong conditions affecting millions of people for which curative treatments are not available. We have identified essential signaling molecules responsible for lymphatic fate specification, sprouting and maturation, opening novel opportunities to target these processes in therapeutic settings.
4. Regulation of Blood-CNS Barrier integrity in health and disease
(Furtado J. et al. PNAS 2024; Boyé K. et al. Nat. Comm. 2022; Del Gaudio et al., Cardiovascular Res, 2024)
Blood vessels in the central nervous system (CNS) form specialized blood-CNS barriers including the blood-brain barrier (BBB) and blood-retina barrier (BRB) that prevent entry of toxins and tightly control the movement of ions, molecules, and cells between the blood and the CNS, thereby ensuring neuronal function and protection. However, the impermeability of the BBB and BRB impedes drug and treatment delivery in various pathologies and the development of tailored therapeutic strategies designed to increase CNS barrier permeability “on-demand” is a critical yet unmet clinical need.
We have identified essential signaling molecules essential for BBB and BRB development and maintenance, opening avenues to increase drug delivery during various CNS diseases.
5. Sphingosine 1-phosphate signaling in vascular development and homeostasis
(Del Gaudio et al., Cardiovascular Res, 2024; Nitzsche et al. Circ Res 2021; Niazi et al Blood Adv 2019; Gazit et al ,Circ Res 2016)
Sphingosine 1-phosphate (S1P) is a bioactive lipid mediator that is produced by erythrocytes and platelets and circulates in plasma bound to high density lipoproteins (HDL) and albumin. S1P plays essential roles in the development and homeostasis of vascular and immune systems through actions on a small family of cognate G protein-coupled S1P receptors (S1PR1-5). We have addressed essential functions and mechanisms of engagement of S1PRs in the vascular system and explored the potential benefits and risks of targeting S1P receptors in cardiovascular disease.
6. Protease signaling in vascular biology and host defense
(Le Gall et al, Blood 2016; Friis S et al, BMC Biol 2017; Kawaguchi M et al, 2020; Szabo et al, PLoS Gent, 2014, Frateschi et al, Nat Commun 2011)
Serine proteases can modulate cellular behavior by proteolytic activation of protease-activated G protein-coupled receptors that carry their own tethered ligands (PARs). PAR signaling plays important roles in vascular development and in hemostasis by coordinating endothelial and platelet responses to activation of the coagulation cascade. We have contributed to the discovery of novel PAR agonists, cross-talk between coagulation proteases and membrane-anchored serine proteases, and roles for protease signaling in epithelial inflammation and cancer. Our current work is focused on roles of protease signaling in placental development and in vascular maturation during later stages of fetal development.