TEAM LEADER : Pierre-Louis Tharaux and Eric Camerer
Mail : pierre-louis.tharaux@inserm.fr /or/ eric.camerer@inserm.fr
PHONE :
THARAUX: +33 6 89 50 29 48
CAMERER: +33 1 53 98 80 48
Localisation :
Tharaux lab: 2nd floor, room 219
Camerer lab: 3rd floor, room 361
DOCTORAL SCHOOL : Ecole Doctorale Bio Sorbonne Paris Cité (BioSPC)

The PL Tharaux and O Lenoir group study the pathophysiological mechanisms of glomerular injury in rare glomerulopathies and common chronic kidney diseases (CKD).
Rapidly progressive crescentic glomerulonephritis (CGN) and focal segmental glomerulosclerosis (FSGS) are severe kidney diseases responsible for irreversible renal failure, which is also a major risk factor for cardiovascular mortality. CGN can even result in a loss of kidney function within days or weeks. Despite the aggressiveness of immunosuppressive protocols applied, treatments against CGN have limited effectiveness. Similarly, there is no approved, specific treatment for FSGS.
We aim to identify druggable pathways and markers of pathogenic glomerular epithelial cells in CGN and FSGS.
Diabetic nephropathy is the leading cause of end-stage renal disease in industrialized countries. Hypertension is a common comorbidity factor of diabetes and diabetic patients with poorly controlled blood pressure commonly present more severe and unclassical renal complications, highlighting the main role of hemodynamic factors to promote glomerular injury. Despite the strong prevalence of diabetic nephropathy worldwide and the emergence of the recent sodium glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists, providing significant but only partial efficacy to prevent cardiovascular events and kidney failure, there are currently no therapies to specifically prevent glomerular cells injury during diabetes and hypertension.
We aim to identify mechanisms of kidney cells tolerance during diabetes and hypertension.
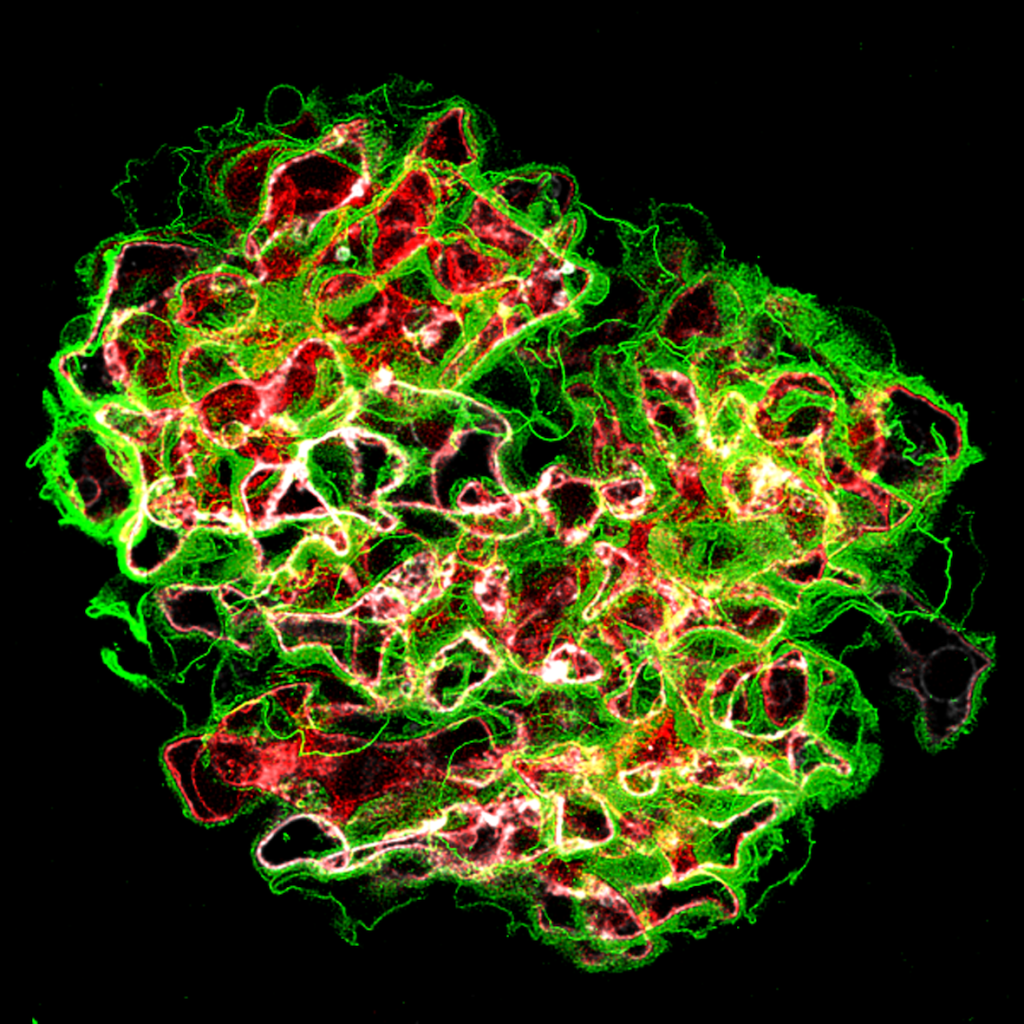
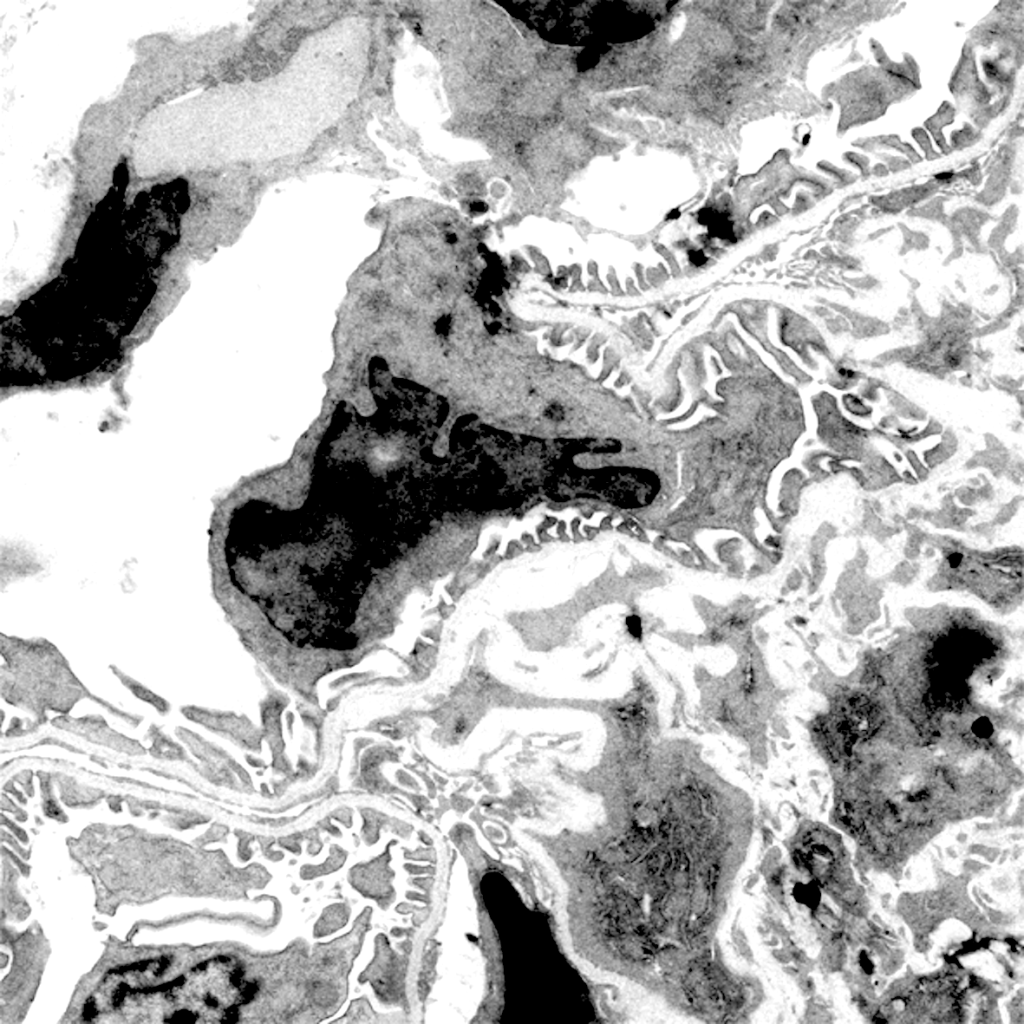
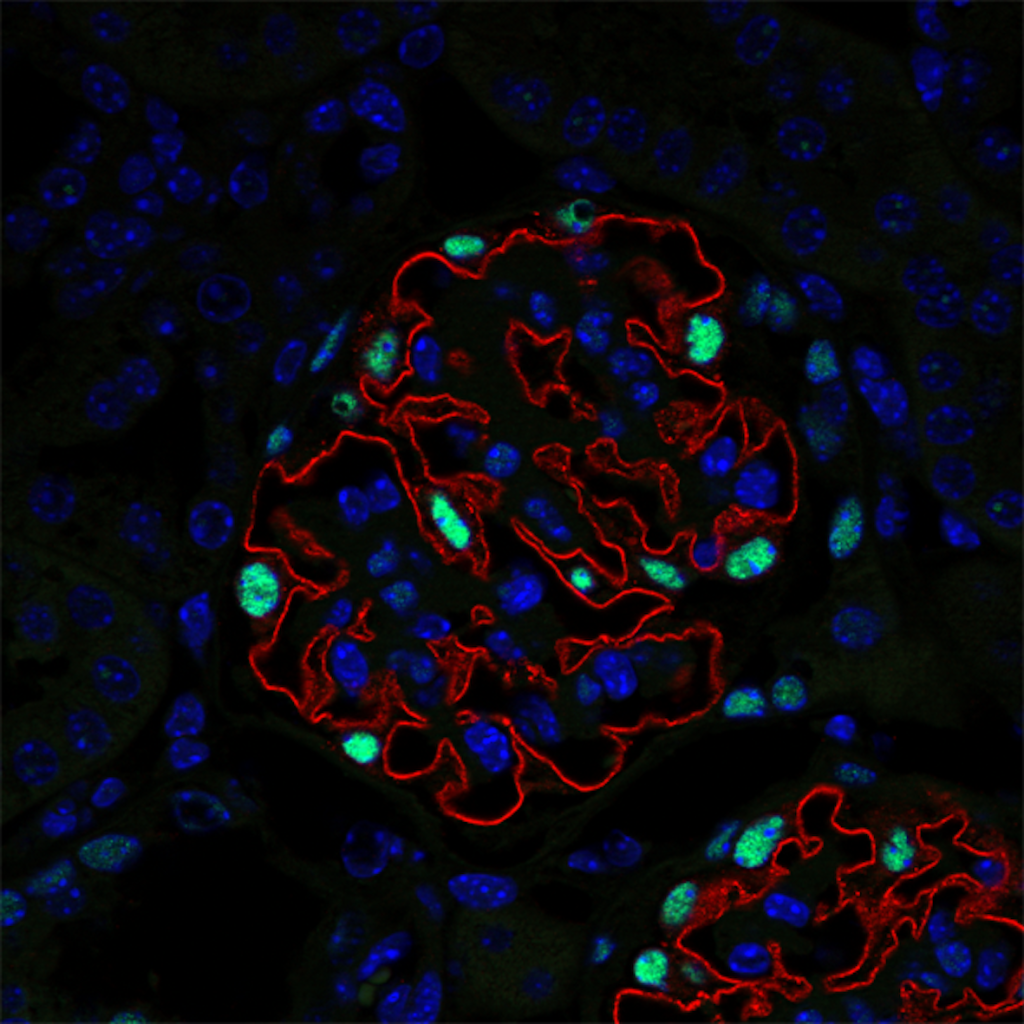
We dissected mechanisms driving the development of extracapillary lesions in FSGS and CGN. We addressed the key question of how parietal epithelial cells (PECs) invade glomerular capillaries, promoting injury and kidney failure. We showed that expression of the tetraspanin CD9 increases markedly in PECs in mouse models of CGN and FSGS and in the kidneys of individuals diagnosed with these diseases. Cd9 gene targeting in PECs prevents glomerular damage in CGN and FSGS mouse models. Mechanistically, CD9 deficiency prevents the oriented migration of PECs into the glomerular tuft and their acquisition of CD44 and β1 integrin expression. These findings highlight a critical role for de novo expression of CD9 as a common pathogenic switch driving the PEC phenotype in CGN and FSGS while offering a potential therapeutic avenue to treat these conditions. Our findings reinforce this paradigm that “activation” of resident glomerular cells play a key role in disease progression and extend the concept by examining interactions between surrounding cellular and molecular systems involved in RPGN.
We uncovered the roles of the ET-1 receptors in myeloid cells in vascular diseases. Myeloid ETB receptors command the concentration of the ET-1 vasoconstrictor in the arterial wall and tissues. Two studies demonstrated the key role for the myeloid ETB receptors in the control of systemic vascular resistance and blood pressure as well as in hypertensive kidney and retina injuries.
We highlighted mechanisms of podocyte injury in chronic kidney disease linking the hypertensive and diabetic environment to the dysregulation of podocyte autophagy. We demonstrated that aberrant calpain activation in podocytes promotes autophagy impairment and subsequent podocyte injury. Calpain inhibition restored podocyte autophagy, limiting podocyte injury in disease models of hypertension and diabetes.
In 2020-21, PL Tharaux co-constructed the CORIMUNO-19 Collaborative Group and thereby contributed to a series of randomised clinical trials to test the efficacy of immunomodulatory drugs in preventing severe forms of COVID-19 and collaborated to investigate the immunopathology of COVID-19. Some of these seminal studies were initiated by B Terrier.